Section number:
SteriStretch®{LRM}
Introduction:
The inserts are manufactured in accordance with the EN ISO 9001 and EN ISO 13485standards. Comply with the requirements of the Medical Device Directive 93/42/EEC. SteriStretch inserts are intended to be used for application to re-usable surgical drapes. The inserts can easily be taped over a tailored contour in the drape. This allows protection of the patient and it creates a sterile and hygienic operating environment. SteriStretch inserts are latex-free sheets with an opening. The sheets are provided with 100% wash-soluble adhesive. During steam sterilization, the adhesive achieves its ultimate adhesive strength and ensures a very strong adhesion between the drape and the insert.
Characteristics:
1.The inserts are 100% latex-free
2.The inserts are not adversely affected by steam sterilization
3.The adhesive is 100% wash-soluble
4.During steam sterilization, the adhesive achieves its ultimate adhesive strength and ensures a very strong adhesion between the drape and the insert
5.The inserts return to their original state after stretching
6.The inserts are biocompatible
Application:
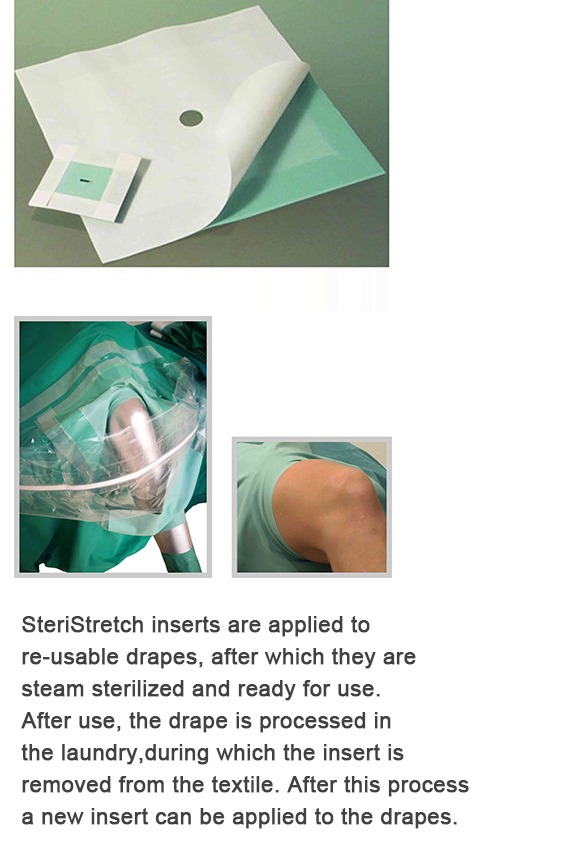
SteriStretch inserts are applied to re-usable drapes, after which they are steam sterilized and ready for use. After use, the drape is processed in the laundry, during which the insert is removed from the textile. After this process a new insert can be applied to the drapes.